Another way chemistry is connected to gardens is through fertilizers. Fertilizers are commonly regarded as materials that are added to the soil or plant to supply one or more elements that are essential for plant growth. Most people are unaware that fertilizers are chemicals, so treat them with care and use protective gloves. Make sure to avoid inhaling odors. The standard fertilizer will have a label reading “20-20-20”, which means that that particular fertilizer has 20% Nitrogen by weight, 20% Phosphorous by weight, and 20% Potassium by weight.
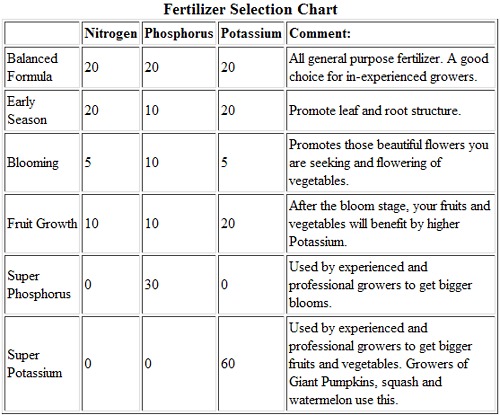
Higher concentrations of Nitrogen should be applied in the early growth stage, as it contributes to leaf root growth which results in a lush, green plant. However, too much Nitrogen can reduce or delay the growth of flowers and fruit, so if your plant is a healthy green, seems to growing well, but doesn’t have flowers, stop adding Nitrogen. You should start using fertilizers that have a higher concentration of Phosphorous when the season moves towards the flowering and fruit set stage. Phosphorous promotes flower growth, root growth, and fruit set and development. Potassium promotes fruit growth. After fruit set, you should start using a high potassium fertilizer. Below is a chart demonstrating possible fertilizer variations.
As mentioned above, there are nitrogen, phosphorous, and potassium fertilizers. There are also quick release/slow release fertilizers, and stabilized nitrogen fertilizers. Anhydrous ammonia is the main component in nitrogen fertilizers, and is produced by the Haber-Bosch process:
3H2+ N2 + heat, pressure, & catalyst = 2 NH3(g) (anhydrous ammonia)
Anhydrous ammonia (82-0-0) is sold as a liquid under pressure and when injected into the soil, undergoes the following reaction:
NH3(g) + H2O <— —> NH4OH <— —> NH4 + OH- —> NO3
The raw material in phosphorous fertilizers is rock phosphate or apatite. They create a superphosphate through the following reactions.
- Apatite + sulfuric acid —> superphosphate.
- Apatite + phosphoric acid triple —> superphosphate
Quick release fertilizers are readily available and are very water soluble. Slow release fertilizers, on the other hand, are less soluble due to the fact that a coating is added to the fertilizer in the granule that delays the rate of dissolution. The advantages of slow release fertilizers include: lower potential to cause injury to plants, higher Nitrogen use efficiency by plants, less subject to volatilization. One disadvantage of slow release fertilizers is that they are fairly expensive. Stabilized nitrogen fertilizers are products mixed with or added to Nitrogen fertilizer materials that either act as a urease inhibitor or a nitrification inhibitor. The fertilizer is said to be stabilized because it is not as subject to losses from volatilization or leaching. This fertilizer is used widely in corn producing areas of the U.S. It is a liquid that is mixed with anhydrous ammonia. In places where the microbial reactions in the soil are already slowed down (perhaps due to cool soil temperatures, as in Alaska) the use of this fertilizer may reduce Nitrogen availability to crops.)
No comments:
Post a Comment